In enzymology, a 3-hydroxyisobutyrate dehydrogenase (EC 1.1.1.31) also known as β-hydroxyisobutyrate dehydrogenase or 3-hydroxyisobutyrate dehydrogenase, mitochondrial (HIBADH) is an enzyme that in humans is encoded by the HIBADH gene.
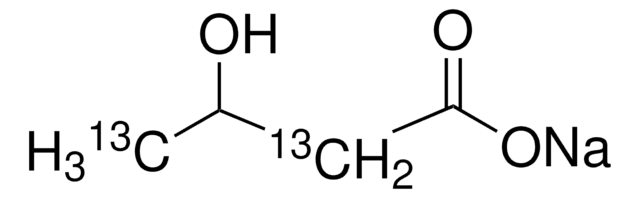
3-Hydroxyisobutyrate dehydrogenase catalyzes the chemical reaction:
- 3-hydroxy-2-methylpropanoate NAD 2-methyl-3-oxopropanoate NADH H
Thus, the two substrates of this enzyme are 3-hydroxy-2-methylpropanoate and NAD , whereas its 3 products are 2-methyl-3-oxopropanoate, NADH, and H .
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD or NADP as acceptor. The systematic name of this enzyme class is 3-hydroxy-2-methylpropanoate:NAD oxidoreductase. This enzyme participates in valine, leucine and isoleucine degradation.
Function
3-hydroxyisobutyrate dehydrogenase is a tetrameric mitochondrial enzyme that catalyzes the NAD -dependent, reversible oxidation of 3-hydroxyisobutyrate, an intermediate of valine catabolism, to methylmalonate semialdehyde.
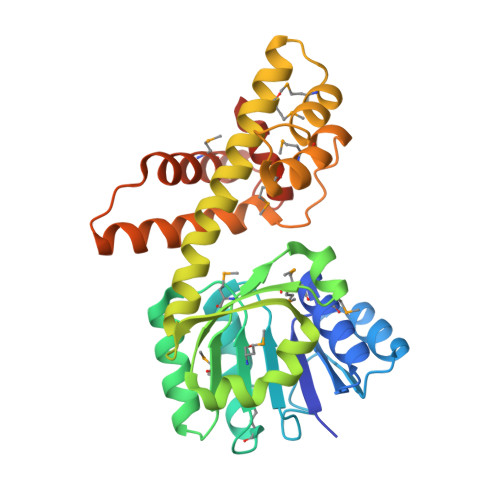
Structural studies
As of late 2007, five structures have been solved for this class of enzymes, with PDB accession codes 1WP4, 2CVZ, 2GF2, 2H78, and 2I9P.
References
Further reading
External links
- Human HIBADH genome location and HIBADH gene details page in the UCSC Genome Browser.
- PDBe-KB provides an overview of all the structure information available in the PDB for Human 3-hydroxyisobutyrate dehydrogenase, mitochondrial